Login
Thoracic Surgery & Interventional Pulmonology
Endobronchial Lung Volume Reduction
Most patients report immediate relief and easier breathing.
Nonsurgical Valve Placement For Patients With Severe Copd / Emphysema
With endobronchial lung volume reduction - a newly FDA-approved breakthrough nonsurgical valve placement procedure for severe emphysema available in Delaware only at ChristianaCare - tiny, one-way valves block off diseased parts of the lung so healthier lung tissue can expand and take in more air.
Clinical studies show that almost immediately patients are able to:
New Treatment, Years Of Experience
ChristianaCare’s Interventional Pulmonology team has years of experience placing endobronchial valves to help patients whose lungs leak air following surgery. Now we are able to use endobronchial valves to help patients with severe emphysema breathe easier and enjoy a higher quality of life without the need for surgery thanks to this newly FDA-approved breakthrough procedure - available in Delaware only at ChristianaCare.
No Incision. No Pain. Immediate Relief.
Here’s How It Works:
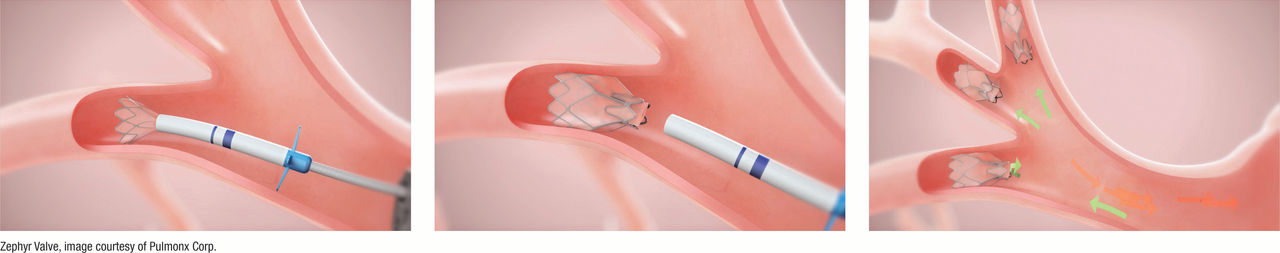
In a procedure known as bronchoscopy that takes about 30 minutes under general anesthesia (where the patient is completely asleep), tiny, FDA-approved valves are guided through a small tube from the nose or mouth to the lungs. No incision is needed.
A tiny camera also guided through the tube helps the interventional pulmonologist place, on average, 4 valves in the airways to block off diseased parts of the lung. The valves reduce hyperinflation, preventing air from becoming trapped in the diseased area of the lung and allowing healthier parts of the lung to take in more air.
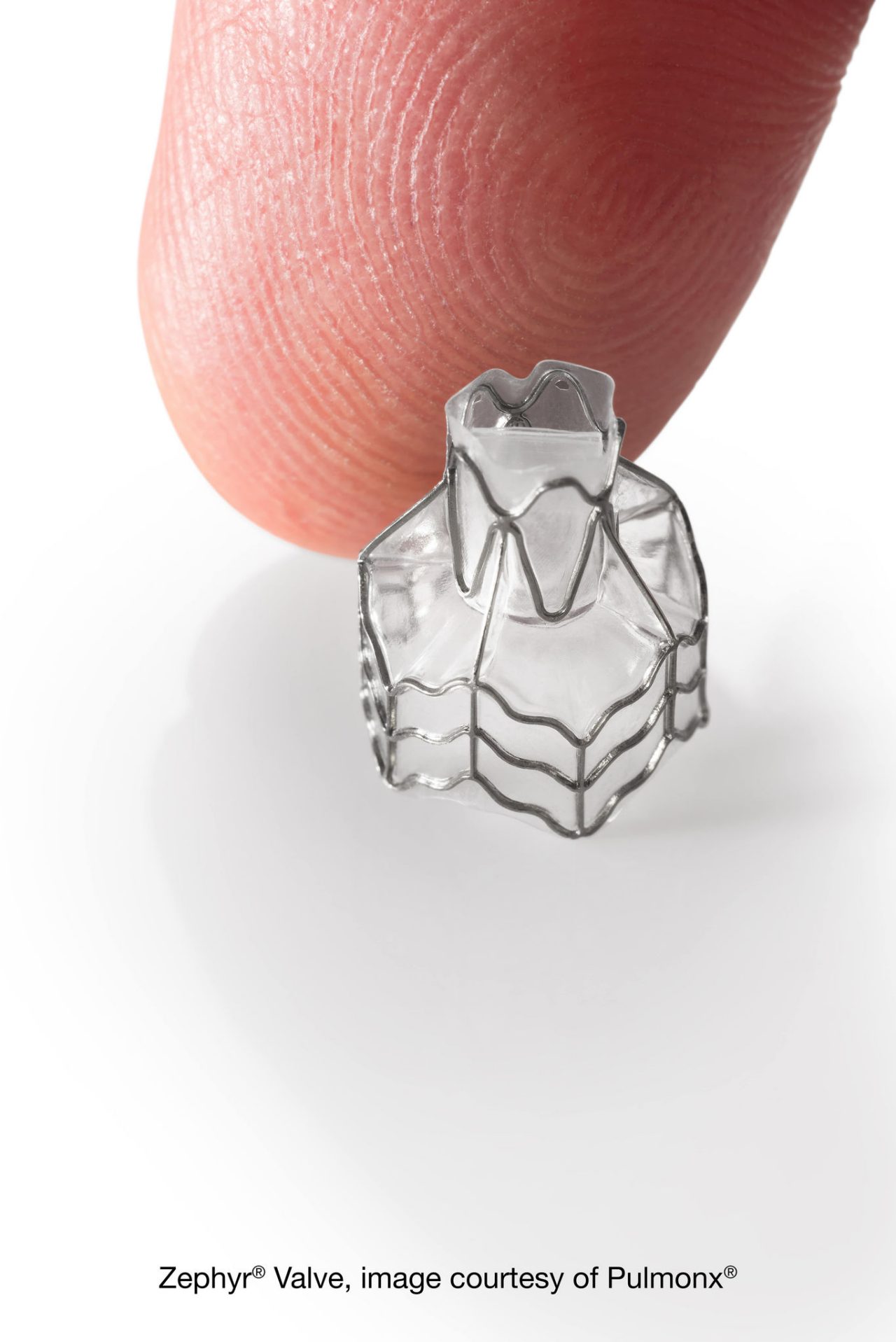
Most patients report immediate relief and easier breathing as soon as they wake up from the procedure. Patients stay in the hospital for approximately 3-5 days for observation following the valve placement.
After the procedure, patients continue to use the medicines prescribed by their doctor for emphysema. Pulmonary rehabilitation helps patients achieve maximum benefit from the valve procedure.
Other program features include a monthly support group and access to new or investigational treatments through participation in industry-sponsored clinical trials.
Endobronchial Lung Volume Reduction May Be Right For You If You:
To learn more, talk to your primary care provider or pulmonologist, or call ChristianaCare’s Interventional Pulmonology team at 302-623-4530.
A simple diagnostic workup that includes pulmonary function testing, a computed tomography (CT) scan and other testing will help to determine if the procedure is right for you.
“My granddaughter doesn’t stop, and now I can keep up with her!”
Thanks to the Interventional Pulmonology experts at ChristianaCare and a few tiny, one-way valves that help her lungs work more efficiently, severe emphysema no longer keeps Joan Trincia from long walks with her family or playing with her granddaughter.
Related Content
Providers & Staff
Contact Us
Helen F. Graham Cancer Center & Research Institute
4701 Ogletown-Stanton Road,
Newark, DE 19713






